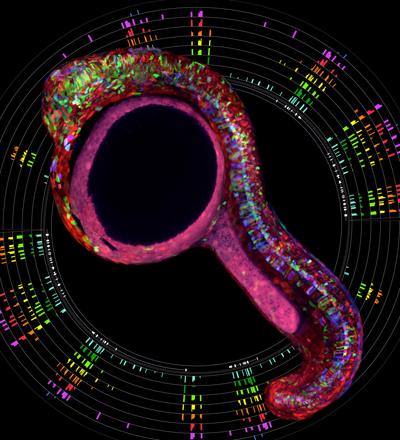
A Zebrabow fish circled by a rainbow of ribosome profiles.
In three papers published in Development, the Schier lab suggests that signals move through developing tissues by hindered diffusion, provides evidence that long non-coding RNAs resemble the 5’ leaders of coding RNAs, and describes tools to apply the Brainbow technology in zebrafish.
In a Hypothesis paper, Patrick Mueller, Katherine Rogers and colleagues address the long-standing controversy of how gradients of signaling molecules form. It had been shown previously that signal gradients induce different cell types depending on the concentration of signal seen by a cell. This morphogen gradient concept is well established but there has been much discussion about how these gradients form: Do the signals move within cells or via long cellular extensions, do they diffuse freely through tissues until they are captured, or do they diffuse but are hindered by interactions with extracellular molecules? Combining mathematical modeling, quantitative imaging and re-analysis of previously published data, the Schier lab suggests that the currently available data are consistent with a model wherein morphogen signals diffuse extracellularly but are slowed down by interactions with other molecules. For example, the morphogens FGF and Nodal move more slowly through tissues than the signaling molecule Lefty because Nodal and FGF (but not Lefty) are thought to interact with extracellular molecules such as heparan sulfate proteoglycans. The regulation of signal diffusion provides a mechanism to shape signal distribution and thereby increase the diversity of cell fates generated during development.
Morphogen transport. Müller P, Rogers KW, Yu SR, Brand M, Schier AF. Development. 2013 Apr;140(8):1621-38. doi: 10.1242/dev.083519.
Read more in Development; Download PDF
In a Techniques and Resources paper, Guo-Liang Chew, Eivind Valen, Andi Pauli and colleagues address the question of whether so-called long non-coding RNAs are truly non-coding. Studies in the past ten years have identified thousands of transcripts that are thought to be non-coding i.e. these RNAs do not serve as the intermediate to generate proteins but function solely as RNAs. Chew et al. used ribosome profiling to sequence the regions of RNAs that are translated, developed a machine learning algorithm that distinguished between 5’ leaders, 3’ trailers and coding regions, and applied this algorithm to classify long non-coding RNAs. They found that dozens of previously annotated “non-coding” RNAs are indistinguishable from coding mRNAs. In addition, they observed that many long non-coding RNAs resemble the 5’ leaders of mRNAs: They carry short translated stretches similar to the translated upstream open reading frames found in many coding mRNAs. These results raise the question of why non-coding RNAs might be translated into proteins that might not have a function. Are these translational events simply noise or do they play a role in RNA regulation and activity?
Ribosome profiling reveals resemblance between long non-coding RNAs and 5′ leaders of coding RNAs. Chew GL, Pauli A, Rinn JL, Regev A, Schier AF, Valen E. Development. 2013 Jul;140(13):2828-34. doi: 10.1242/dev.098343. Epub 2013 May 22
Read more in Development; Download PDF
In another Techniques and Resources paper, Albert Pan and colleagues introduce several methods and transgenic lines that allow the application of the Brainbow technology developed by Livet, Sanes, and Lichtman to zebrafish. Pan et al. show that that the combinatorial expression of multiple fluorescent proteins can label cells in dozens of different colors. They demonstrate that this Zebrabow (ZEBRAfish + brainBOW) technology can be used to distinguish and follow cells over days in live fish. The lines have already been distributed to more than 100 laboratories and will help accelerate anatomical and developmental studies in zebrafish. Similar to the mouse Brainbow lines, the Zebrabow lines have garnered a lot of attention for the visually stunning images they produce. Unexpectedly, there are also some caveats to having pretty Zebrabow pictures around for all to see. Albert’s three-year old daughter visited the lab a while ago. He wanted to impress her with an axon beautifully labeled by green fluorescence. She took a look in the microscope and asked, “Where are all the other colors?”
Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Pan YA, Freundlich T, Weissman TA, Schoppik D, Wang XC, Zimmerman S, Ciruna B, Sanes JR, Lichtman JW, Schier AF. Development. 2013 Jul;140(13):2835-46. doi: 10.1242/dev.094631
Read more in Development; Download PDF